Project INSPECT
We have developed a CMOS biochip with which the prostate-specific antigen can be quantitatively detected. The chip achieves the specifications required for clinical PSA tests according to Rili-BÄK for early cancer detection.
The goal: rapid, precise testing for prostate cancer diagnosis
During diagnosis and care of prostate cancer, PSA concentration (prostate-specific antigen) is determined at all stages: early recognition, exploratory investigation, treatment monitoring and follow-up care. Even a few nanograms PSA per millilitre blood (ng/ml) are an indication of whether and which further tests and treatment are needed. Higher PSA concentrations may be a sign of cancer or its return. The physician takes blood and sends it away for analysis. The PSA test results usually take a few days to come back. Not only for PSA concentrations, science has been working on one new point-of-care (PoC) testing system after another which may obviate costly, time-consuming lab tests carried out on huge, complex apparatus and reduce to a minimum the usually very worrying wait for the patient.
In many cases, such solutions involve test strips which change colour enabling the doctor to assess concentrations in the consultation room. The colour changes vary in intensity and are caused by biochemical reaction. There has been a move in recent years towards using PoC devices with photodetectors or a camera for concentration testing.
Apparatus of this kind still labours under the disadvantage of inaccuracy in comparison with laboratory testing. In the case of PSA detection, the ”Rili-BÄK“ (the guidelines published by the German Medical Association (Bundesärztekammer) for quality assurance in medical laboratory tests) prescribe a lower detection limit of 0.2 ng/ml and a range up to 50 ng/ml. Confidence in the results depends on a coefficient of variation (CV) which is at most 15.5%. Measurements taken with a PoC reader and PSA test strip combination developed for reference purposes in the current research project, however, failed to reach these standards.
Chip-based prototype detects and measures PSA at less than one nanogram per millilitre
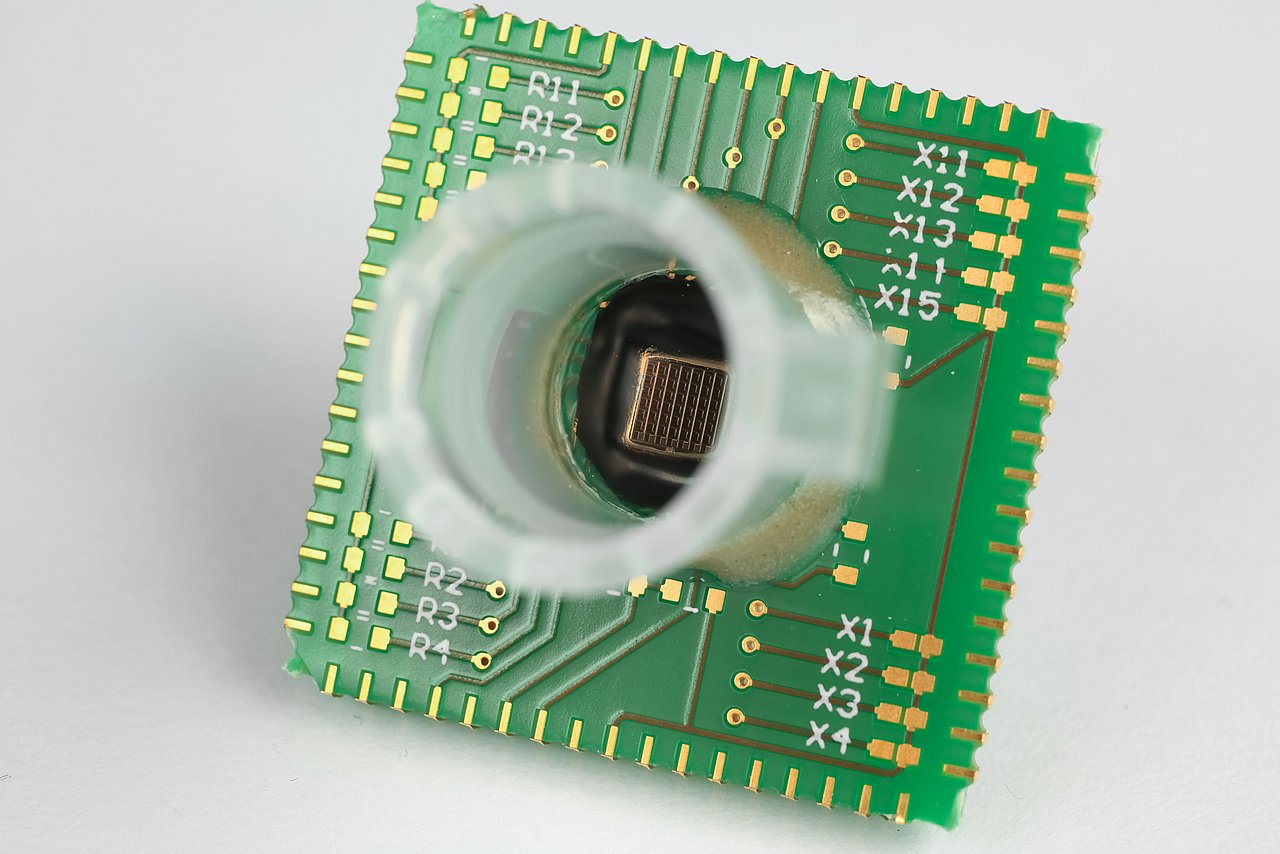
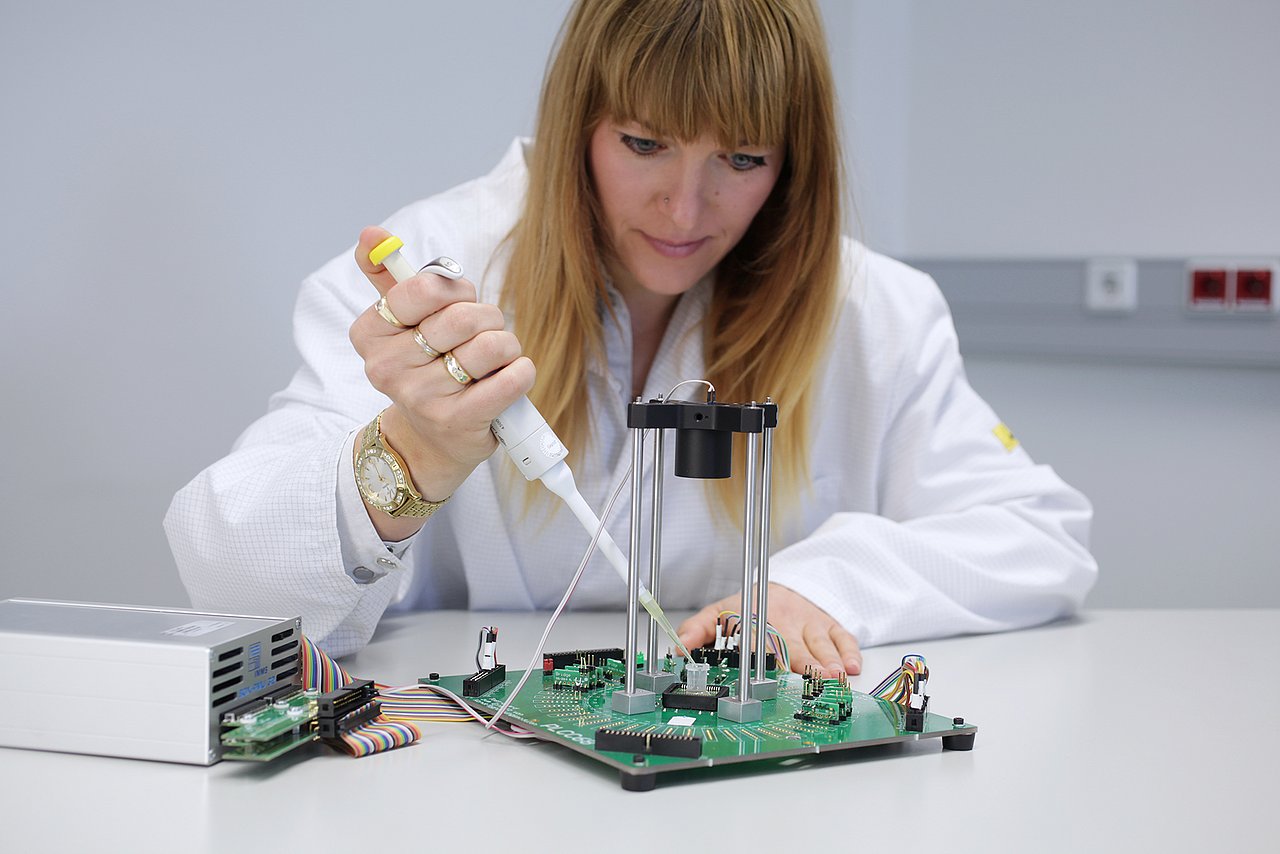
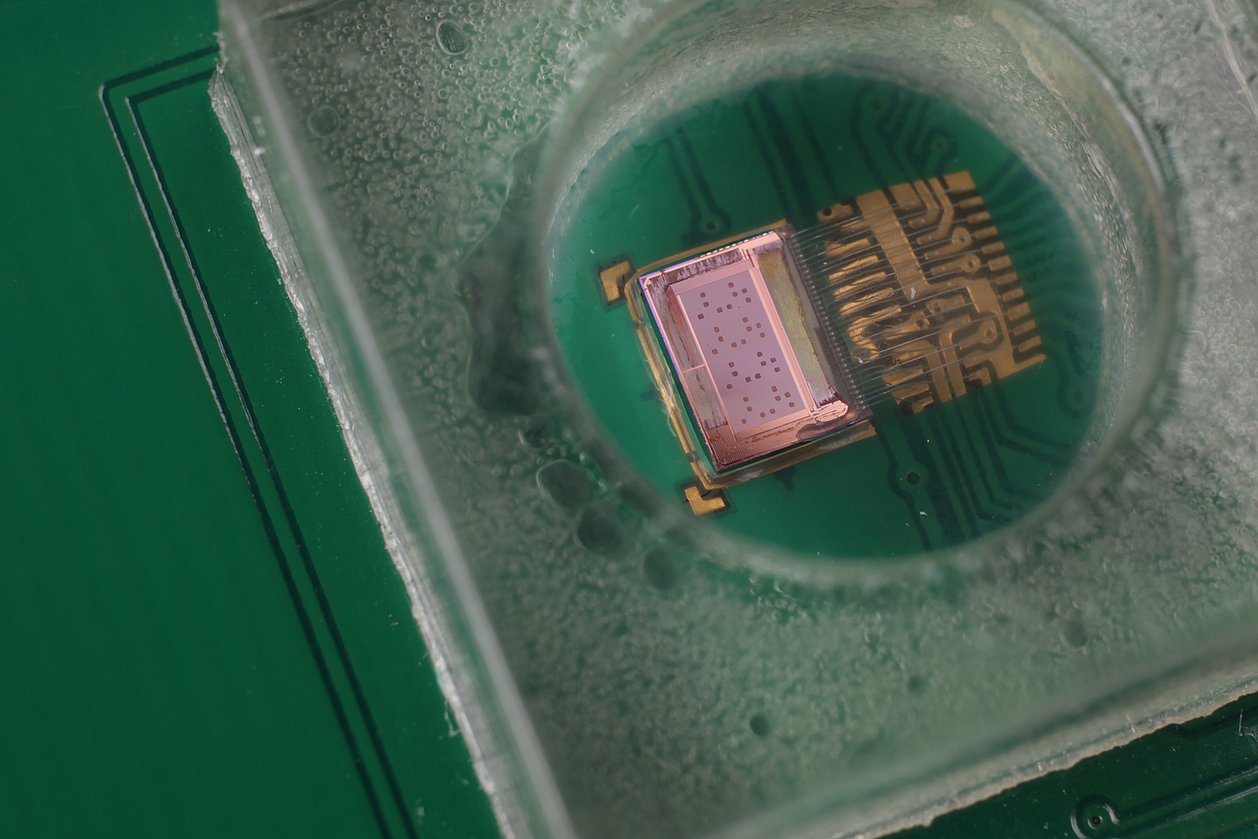
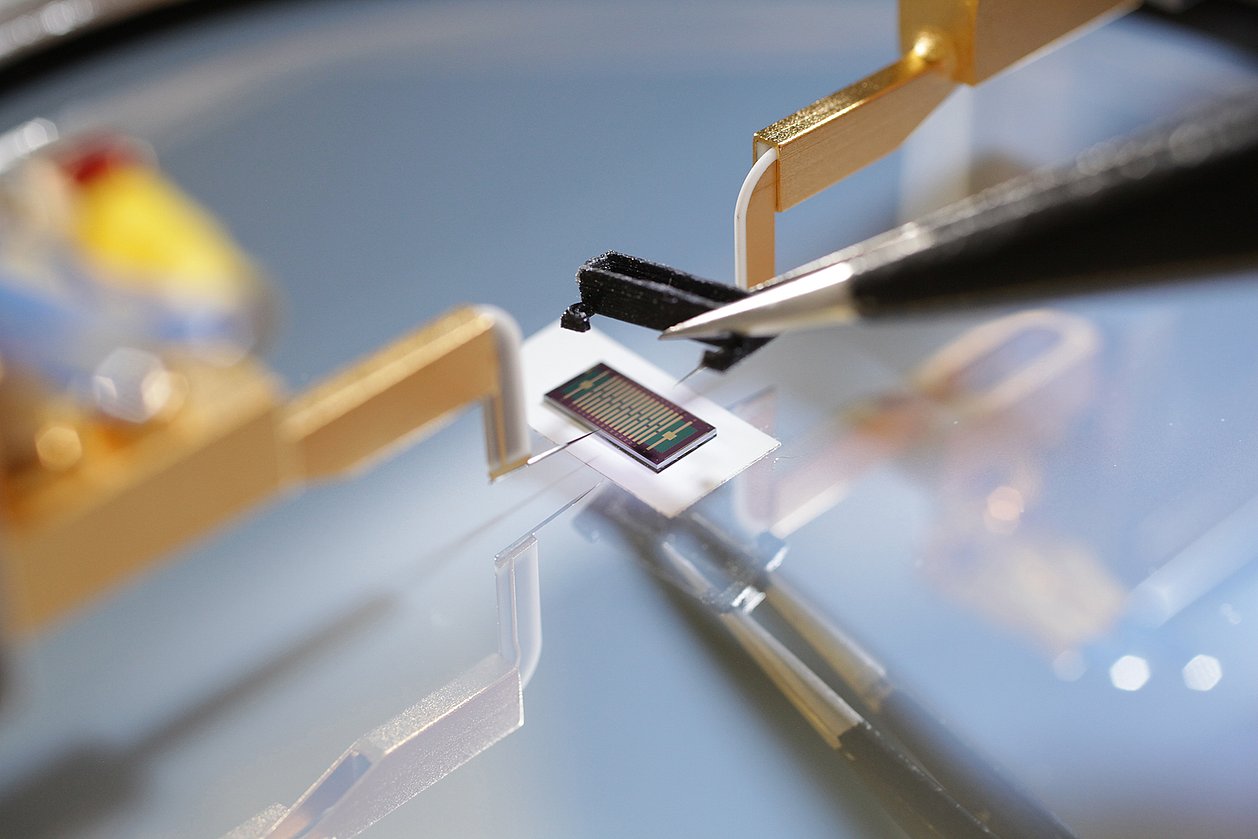
In the INSPECT project, IMMS designed and developed a photometer as a portable reader in combination with a replaceable opto-electronic CMOS biochip and produced a demonstration model. Instead of the test strip employed to date in certain PoC systems with camera, the new version measures the biochemical reaction without a gap between photometer and sample, which is placed directly onto a microelectronic chip. IMMS’ partner in the project, the company Senova Gesellschaft für Biowissenschaft und Technik mbH, undertook the tasks firstly of providing the chip surface with its biochemical functions using immobilised prostate-specific antibodies and secondly of using the prototype to measure samples with various PSA concentration levels. If the sample contains PSA, this is shown by biochemical reactions which darken the sample to an extent that varies with the concentration and with photometry and electronics the differences in brightness are measured. It is a world first for the Senova and IMMS partnership that the presence of PSA on a CMOS biochip can be proven with a lower detection limit of 0.1 ng/ml. This degree of accuracy fulfils the requirements for clinical PSA tests set by ”Rili-BÄK“. The total time required for the test with the CMOS biochip is 14 minutes, there is digital output of the results and these can be processed on a computer connected to the diagnostic device.
For the test and characterisation of the developed integrated circuits, we used and further developed our modular and mobile test systems.
Acronym / Name:
INSPECT / Microelectronic diagnostic platforms for personalised cancer research and micro-bioreactorsDuration:2016 – 2019
Application:
Life Sciences|cancer screenings| point-of-care systems (POC)| in-vitro diagnostics (IVD)Research field:Integrated sensor systems
Related content
Reference
Dr. Friedrich Scholz, Senova
“IMMS demonstrated great commitment in responding to the huge challenges on the development of the point-of-care test. Our experience demonstrates that IMMS application-oriented analyses, understands and models the biochemical processes. Furthermore, the colleagues implement the specifications with their integrated system design and are flexible in adapting the systems as necessary.”

PSA-Nachweis direkt am Point-of-Care
Alexander Hofmann1. Michael Meister1. Alexander Rolapp1. Peggy Reich1. Friedrich Scholz2. Eric Schäfer1.in medical-design, Innovative Produkte und Lösungen in der Medizintechnik, 02/2021, S. 29-32, shop.weka-business-communication.com/medical-design-dsb/Einzelhefte/medical-design-02-2021-Digital.html, www.medical-design.news
1IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, 98693 Ilmenau, Germany. 2Senova Gesellschaft für Biowissenschaft und Technik mbH, Weimar, Germany.Light-Controlled Photometer with Optoelectronic CMOS Biochip for Quantitative PSA Detection
Alexander Hofmann1. Michael Meister1. Alexander Rolapp1. Peggy Reich1. Friedrich Scholz2. Eric Schäfer.2020 IEEE International Symposium on Circuits and Systems (ISCAS), Sevilla, 2020, pp. 1-5, DOI: doi.org/10.1109/ISCAS45731.2020.9180796
1IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, 98693 Ilmenau, Germany. 2Senova Gesellschaft für Biowissenschaft und Technik mbH, Weimar, Germany.An Integrated CMOS Photodiode Array for Highly Sensitive Photometric Diagnostics
Alexander Hofmann1. Michael Meister1. Susette Germer1. Friedrich Scholz2.2018 15th International Multi-Conference on Systems, Signals & Devices (SSD), Yassmine Hammamet, Tunisia, 19-22 March 2018, pp. 1465-1470. DOI: doi.org/10.1109/SSD.2018.8570541
1IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, 98693 Ilmenau, Germany. 2Senova Gesellschaft für Biowissenschaft und Technik mbH, Weimar, Germany.Entwurf, Aufbau und Test eines photometrischen Messsystems auf Basis eines CMOS-Sensorarrays für die schnelle und zuverlässige Vorort-Diagnostik von Prostatakrebs
Friedrich Scholz1. Alexander Hofmann2.Workshop „Neue Sensorlösungen für Biologie und Medizin“, 23. Oktober 2018, Anwendungszentrum Mikrosystemtechnik Erfurt
1Senova Gesellschaft für Biowissenschaft und Technik mbH, Weimar, Germany. 2IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, 98693 Ilmenau, Germany.
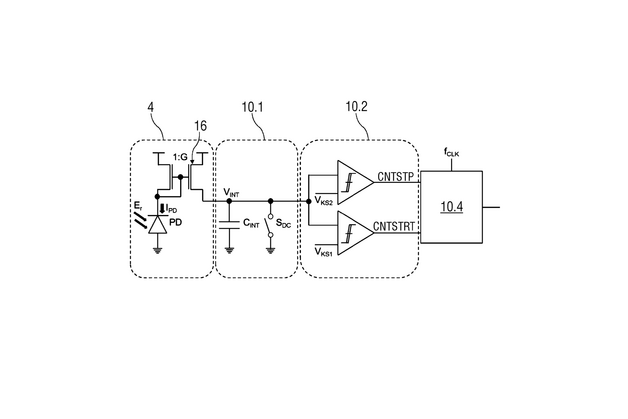
Patent
DE 10 2018 218 122
Device and method for analysing biological, chemical and biochemical substances
Press release,
Mobile rapid tests for prostate and colon cancer – microelectronics measures antigen concentration
IMMS demonstrates functional model at MEDICA trade fair, Nov 13th – 16th, hall 3/G60

Press release,
IMMS presents three developments at MEDICA 2017
Nov 13th – Nov 16th 2017, Düsseldorf (Germany), joint booth DiagnostikNet BB, hall 3/G60
Contact
Contact
Eric Schäfer, M. Sc.
Head of Microelectronics / Branch Office Erfurt
eric.schaefer(at)imms.de+49 (0) 361 663 25 35
Eric Schäfer and his team research Integrated sensor systems, especially CMOS-based biosensors, ULP sensor systems and AI-based design and test automation. The results are being incorporated into research on the lead applications Sensor systems for in-vitro diagnostics and RFID sensor technology. It will assist you with services for the development of Integrated circuits and with IC design methods.
Contact
Dipl.-Ing. Michael Meister
Head of Industrial Electronics and Measurement Technology
michael.meister(at)imms.de+49 (0) 3677 874 93 20
Michael Meister is your contact for testing services, the development of test methodologies, and long-term measurements. He answers your questions on Modular and mobile test systems that we develop in our research in Smart distributed measurement and test systems as well as about testing and characterisation of integrated sensor systems. He is responsible for the test equipment at IMMS and will support you in the validation of ASIC and MEMS developments.
Funding
The INSPECT project was funded by the “Land” of Thüringen and the European Union under the reference 2015 FE 9159.