Press releases
Mobile rapid tests for prostate and colon cancer – microelectronics measures antigen concentration
IMMS demonstrates functional model at MEDICA trade fair, Nov 13th – 16th, hall 3/G60
9th November 2017. At the MEDCIA trade fair in Düsseldorf, Germany, IMMS will present a mobile microelectronic testing system for early diagnosis of prostate and colon cancer. Via live-demo of a functional model IMMS will illustrate the detection principle the current work is based on and which is carried out in the INSPECT project with four partners in Thüringen, Germany. It is the role of IMMS to develop the application-specific microelectronics, focussing on signal processing, particularly in the case of very weak signals, and efficient noise suppression for these. These chips are currently being used by the Senova Gesellschaft für Biowissenschaft und Technik mbH to conduct extensive test series with biological samples. The foundation is Senova’s expertise in immunological assays and in biochemical surface functionalisation of chips. Thanks to the combination of immunological testing methods with microelectronics it becomes possible to early, precisely and reliably verify lowest concentrations of cancer biomarkers.
Present rapid tests no more than qualitative
There are certain types of cancer for which the patient’s doctor can test rapidly on the spot, obtaining an immediate result and saving costly, time-consuming lab tests. The present state of the art includes the strip test on which antibody molecules have been deposited. These seeker molecules have a colour marker and will bind the target molecule to them, producing a result within 5 or 10 minutes: the answer is ”yes“ or ”no“ according to the presence of a coloured line. However, varying strength in the colour of this line is not information that the user can interpret. It is also possible for a very pale line to be overlooked.
Future quantitative rapid tests for exact diagnosis
On the other hand, if it were possible to measure the exact concentration of certain molecules in sample fluids, reliable diagnosis could be achieved. Particularly in the case of cancer of the prostate, there would be a great improvement if the on-site diagnostics could include such quantitative analysis. Although the presence of PSA (prostate-specific antigen) may be an indicator of cancer, it is constantly being produced in the male body. A man less than 50 years old will have a PSA concentration of less than 2.5 ng/ml (the unit is in thousand-millionths of a gram, i.e. in nanograms, per millilitre). At over 70, a man will have a level around 6.5 ng/ml. It is possible for these values to vary independently of age; the cause may be inflammation, mechanical irritation or cancer. If there is a carcinoma developing, the patient’s PSA concentration will be rising continuously. If the PSA concentration could be measured at regular intervals, reliable early diagnosis and early treatment would result.
Microelectronics to measure antigen concentrations in colon and prostate cancer
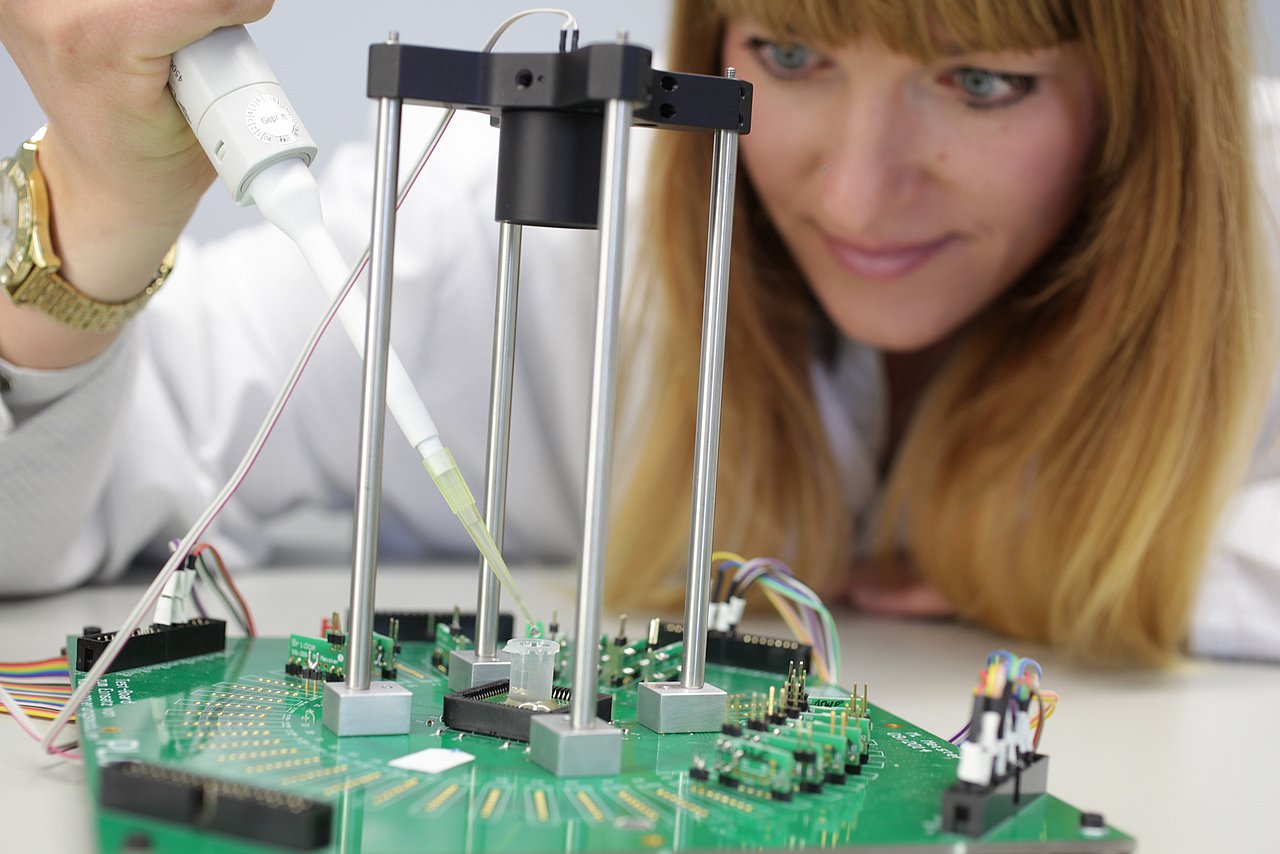
The biotechnical principles on which the new test system will be based are comparable with those for the strip test. The interaction between antibody and antigen is intended to enable detection of analytes in a sample: PSA in the case of prostate cancer and haemoglobin in the case of colon cancer. To meet the diagnostic needs, the chip must be capable of recognising concentrations of antigens in the range of one nanogram per cubic centimetre. These low concentrations induce very weak fluctuations in the luminous intensity, between 0.01 Bel and 1 Bel.
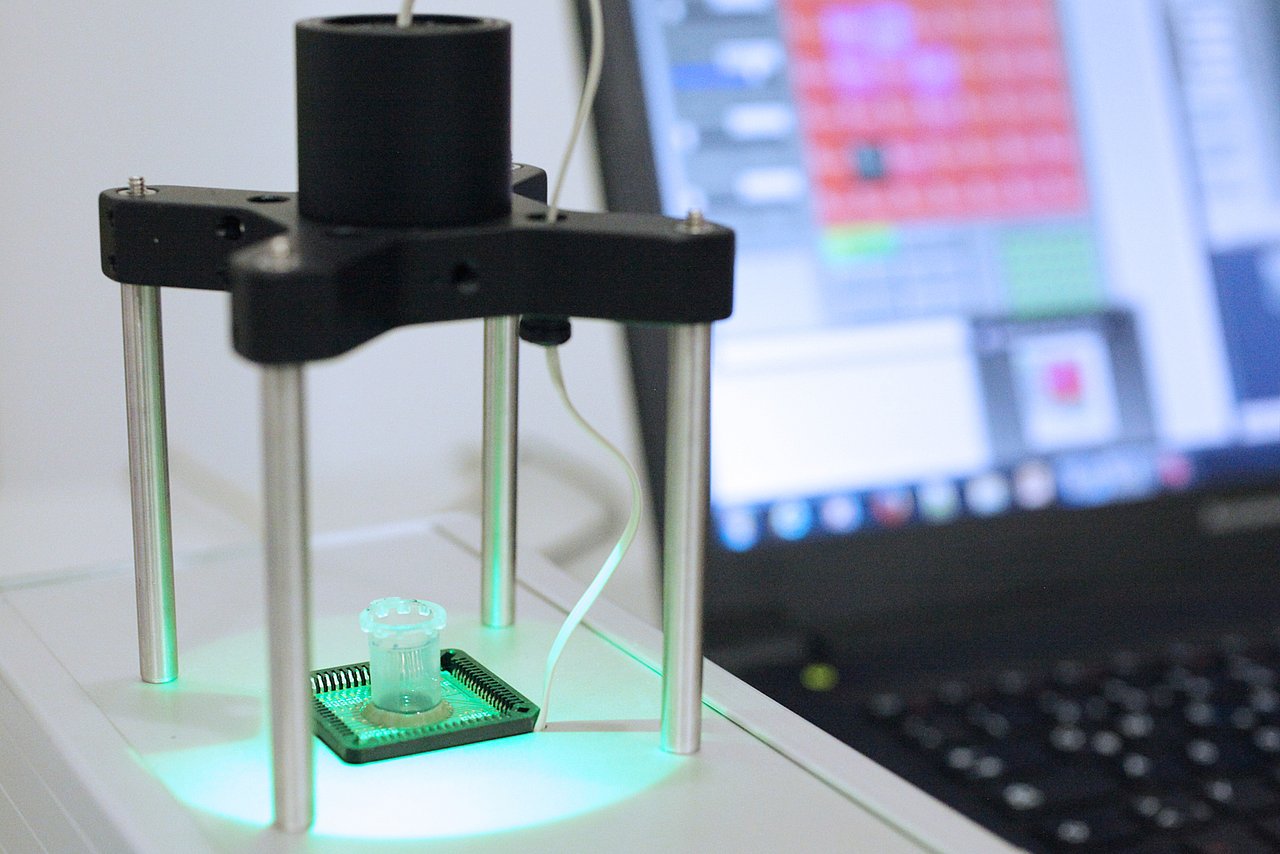
IMMS has thoroughly investigated the technical feasibility of achieving this level of accuracy for the purpose of cancer diagnosis. As a first step an already available chip originally intended for detecting infectious diseases was evaluated to see how it would visualise varying levels of brightness in sample fluids where the concentration of particles is known. TMB (tetramethylbenzidine) substrate solutions were deposited on this chip and the TMBs were enriched with HRP (the horseradish peroxidase enzyme). The chemical reactions which took place turned the fluids blue – the lower the concentration, the more slowly. The chips were used to measure the gradual attenuation of light due to staining over time and tiniest HRP quantities have already been verified.
For this purpose, 4 sample solutions were used which contained 0 ng/ml, 0.2 ng/ml, 1 ng/ml and 5 ng/ml of HRP respectively. For each sample, on addition of the specified HRP concentration, a brightness value was recorded for each second over a period of 600 seconds. These recordings proved that the alterations in brightness were indeed in the range 0.01 Bel to 1 Bel and the visualised differences in the reaction processes were consonant with expectations. It is intended to rely on this preparatory work in developing correlations for the analyte concentrations which would prove the presence of PSA and haemoglobin to specify requirements for the new chip design.
View on the intermediate state of the mobile microelectronic-based test system
„A point-of-care diagnostic system could be prototyped, allowing bio-medical analyses to be carried out at the premises of the Senova GmbH. The high resolution of the signals and the accurate signal detection made it clear that simple, quick, reliable diagnosis and monitoring of certain cancers is possible,“ says Dr. Friedrich Scholz, Senova Gesellschaft für Biowissenschaft und Technik mbH, about the state of development. „The system is currently being tested for prostate and colon cancer diagnostics. In the future the various sensors on the chip should allow the analysis of a range of parameters so that cancer diagnosis will become ever more reliable and certain and so that the monitoring of individual therapies will become possible.“
Related content

Project
INSPECT
We have developed a CMOS biochip with which the prostate-specific antigen can be quantitatively detected. The chip achieves the specifications…
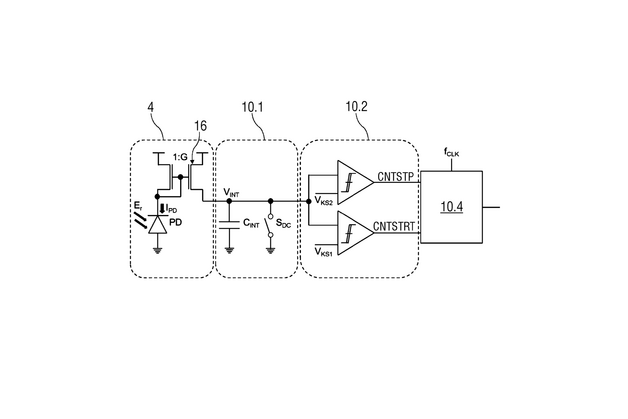
Patent
DE 10 2018 218 122
Device and method for analysing biological, chemical and biochemical substances
Contact
Contact
Dipl.-Hdl. Dipl.-Des. Beate Hövelmans
Head of Corporate Communications
beate.hoevelmans(at)imms.de+49 (0) 3677 874 93 13
Beate Hövelmans is responsible for the text and image editorial work on this website, for the social media presence of IMMS on LinkedIn and YouTube, the annual reports, for press and media relations with regional and specialist media and other communication formats. She provides texts, photographs and video material for your reporting on IMMS, arranges contacts for interviews and is the contact person for events.