Project KODIAK
IMMS researches image sensors for chemiluminescence assays with Thuringian industry and institutes from Erfurt-Südost and Jena
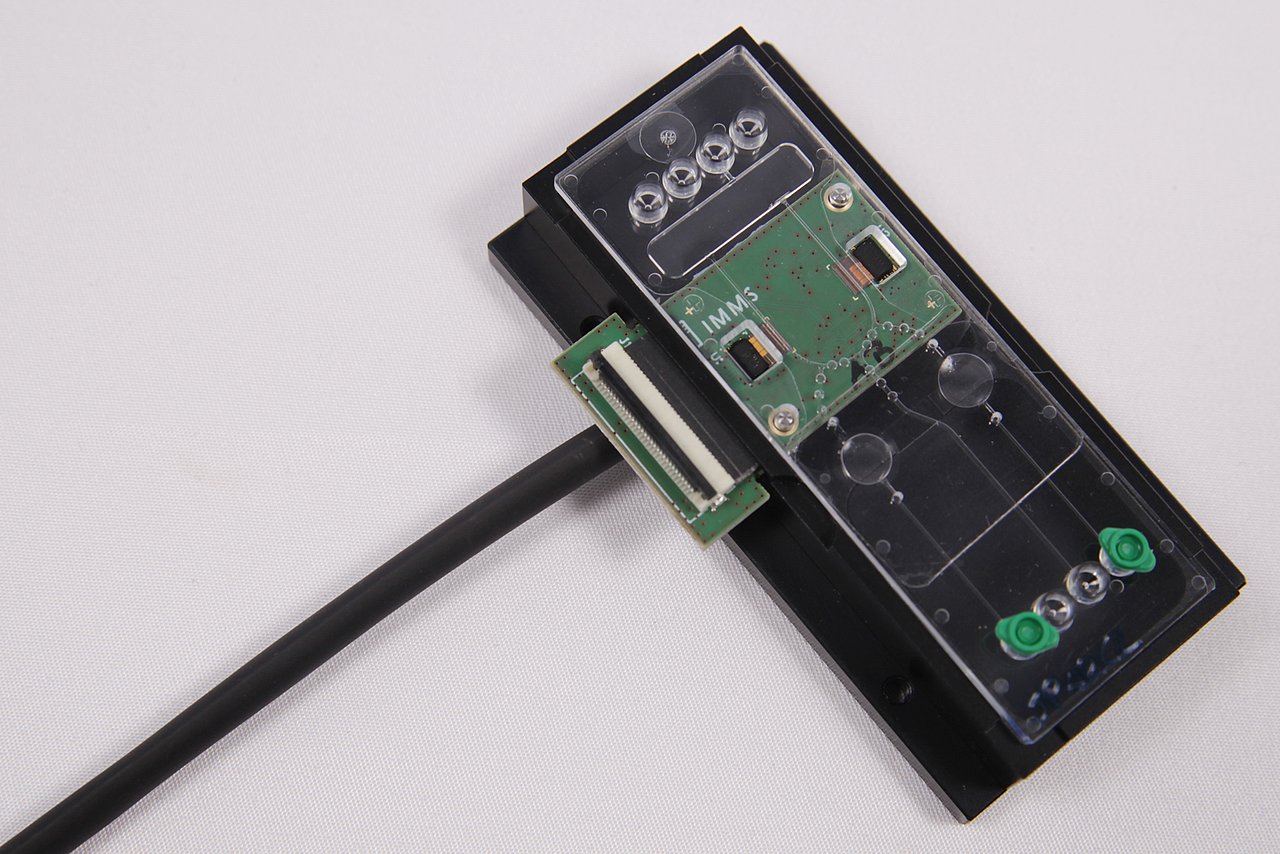
Lab-on-chips (LOC) integrate various laboratory diagnostic procedures for sample preparation and analysis on one chip and can process and evaluate patient samples automatically. The disposable cartridges are often no larger than a credit card, their device platforms small and thus optimal for diagnosis or first aid in medical practices or for point-of-care diagnostics in general. Results can thus be provided more cheaply, faster and earlier than conventional analyses in a medical laboratory. In KODIAK, electronic, optical and fluidic components and associated integration techniques are being developed to open up LOC-based point-of-care diagnostics for further use cases.
Highly sensitive image sensors with SPADs for chemiluminescence assays
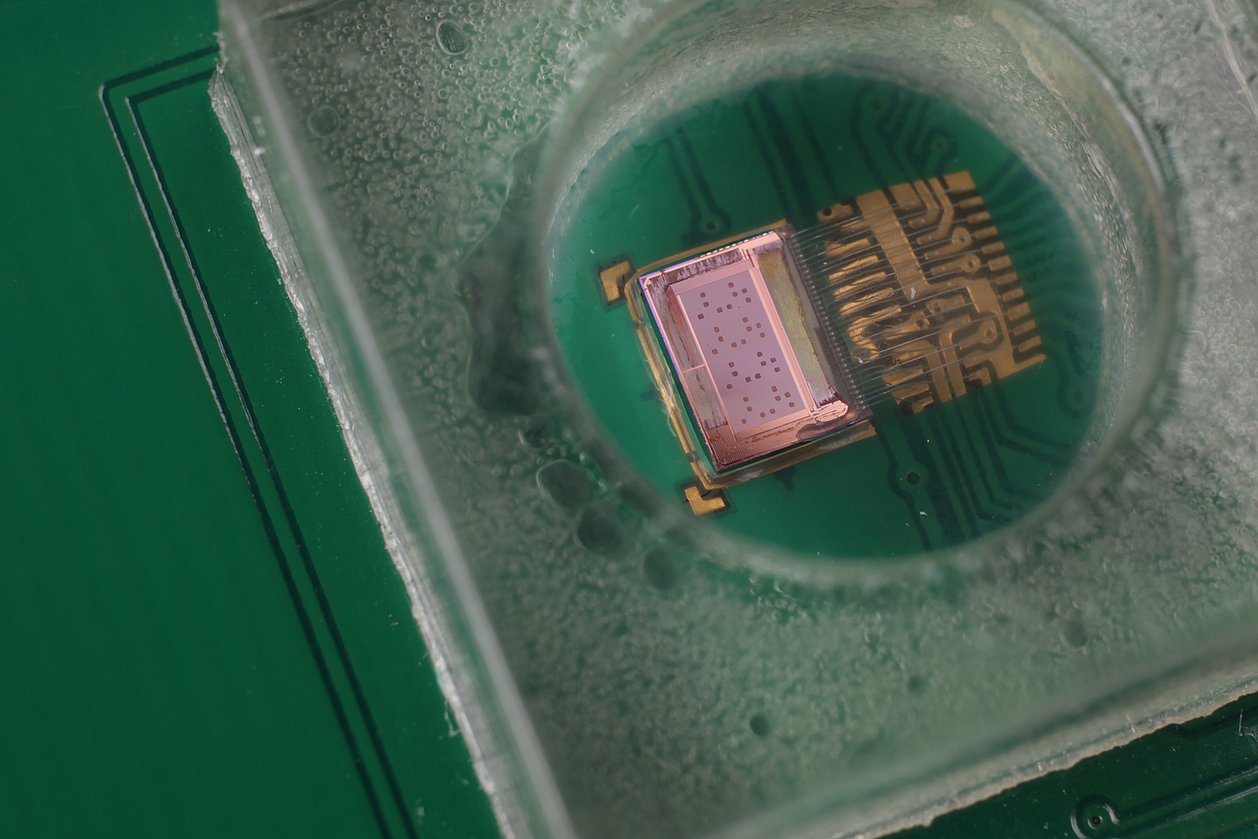
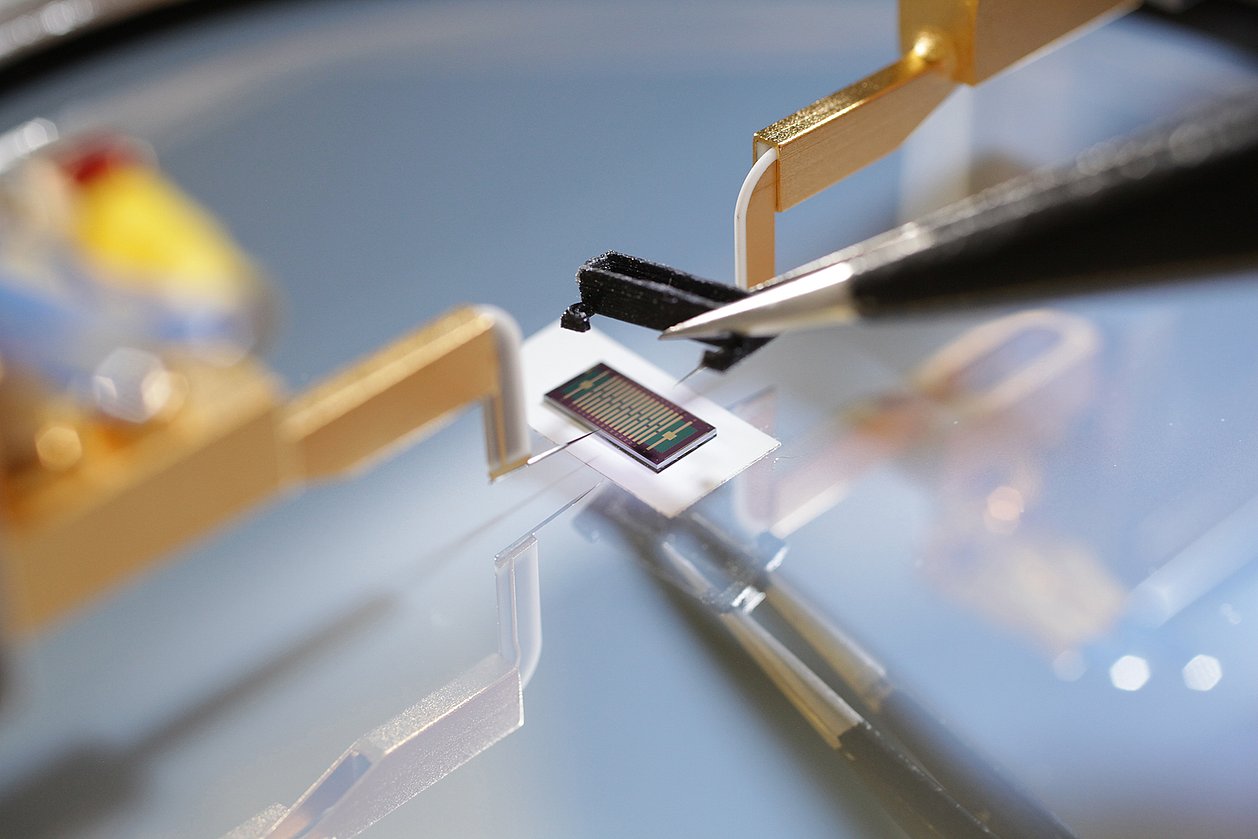
IMMS is contributing its expertise on CMOS-integrated optical sensors to the project. We are investigating how the new single photon avalanche diodes (SPADs) of our project partner X-FAB can be used in highly sensitive image sensors for chemiluminescence LOC assays. We are developing a line sensor for reading chemiluminescence in microfluidic cartridges. In addition, hardware and software modules are also being developed to evaluate the sensor chip in an application-oriented manner.
First application – diagnosis of the frequently lethal CR syndrome
The development is essentially driven by enabling rapid and cost-effective diagnoses for cytokine release syndrome (CRS) and at the same time demonstrating the suitability of the technical project results in medical applications. CRS occurs as a serious side effect in a variety of diseases and therapies, such as immunotherapy, graft-versus-host disease, sepsis and infectious diseases such as Covid-19, and is often lethal. Depending on the study, for example, between about 20 – 45% of patients treated with CAR T-cell therapy in oncology suffer from CRS, as do the majority of corona patients who die. CRS must therefore be recognised and treated as quickly as possible. Currently, diagnosis is symptomatic, i.e. delayed and unspecific, and via blood tests and thus invasive, which excludes e.g. online monitoring. With the new image sensors, serious disease processes are to be detected earlier, clinical capacities are to be used more effectively and the medical care of patients is to be strengthened.
Acronym / Name:
KODIAK / Components and modules for improved optical diagnosticsDuration:2021 – 2023
Application:
Life Sciences|Life sciences| in-vitro diagnostics| cytokine release syndrome (CRS)| Corona| Covid-19Research field:Integrated sensor systems
Related content
Point-of-care solution for rapid monitoring of cytokine release syndrome by chemiluminescence cytokine detection on sensitive Single Photon Avalanche Diodes (SPAD) sensors
A. Lopes1. P. Scholz1. S. Allelein1. A. Kölsch1. E. Schäfer2. M. Wiener2. B. Saft2. N. Isserstedt-John3. D. Kuhlmeier1.18. Research Festival for Life Sciences, 30. Januar 2025, Leipzig
1Fraunhofer-Institut für Zelltherapie und Immunologie IZI, Leipzig. 2IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, Ehrenbergstraße 27, 98693 Ilmenau, Germany. 3microfluidic ChipShop, Jena.Enhancing Chemiluminescence-Detection with Dark-Count Rate Optimization Strategies for SPADs in Conventional CMOS Technologies
Benjamin Saft1. Alexander Zimmer2. Maximilian Wiener1. Mirjam Skadell2. Eric Schäfer1.ISSW 2024, The International SPAD Sensor Workshop & the SPAD SENSOR SCHOOL, Trento, Italy, June 3 - 6, 2024
1IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, Ehrenbergstraße 27, 98693 Ilmenau, Germany. 2X-FAB Semiconductor Foundries GmbH, Erfurt, Germany.Integration of Chemiluminescence-based SPARCL Assay on Microfluidic Platform with SPAD-based CMOS Line Sensor IC for Rapid Cytokine Detection in CRS
Benjamin Saft1. Biljana Gjurova. Pia Scholz. Alexander Zimmer. Mirjam Skadell. Eric Schäfer1. Susann Allelein.POCT Meeting 2024, 17. - 18. April 2024, Leipzig
1IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, Ehrenbergstraße 27, 98693 Ilmenau, Germany.SPAD-Based Sensor IC for chemiluminescence assays in microfluidic channels
Alexander Zimmer1. Benjamin Saft2. Maximilian Wiener2. Jakob Hampel2. Mirjam Skadell1. Eric Schäfer2.Conference of Science & Technology for Integrated Circuits (CSTIC), Shanghai, China, March 17-18, 2024, DOI: doi.org/10.1109/CSTIC61820.2024.10531988
1X-FAB Global Services GmbH, Erfurt, Germany. 2IMMS Institut für Mikroelektronik- und Mechatronik-Systeme gemeinnützige GmbH, Ehrenbergstraße 27, 98693 Ilmenau, Germany.

Event,
CSTIC 2024
Conference of Science & Technology for Integrated Circuits (CSTIC) in conjunction with SEMICON China 2024
Contact
Contact
Eric Schäfer, M. Sc.
Head of Microelectronics / Branch Office Erfurt
eric.schaefer(at)imms.de+49 (0) 361 663 25 35
Eric Schäfer and his team research Integrated sensor systems, especially CMOS-based biosensors, ULP sensor systems and AI-based design and test automation. The results are being incorporated into research on the lead applications Sensor systems for in-vitro diagnostics and RFID sensor technology. It will assist you with services for the development of Integrated circuits and with IC design methods.
Funding
The KODIAK project was funded as part of the European Union's response to the COVID-19 pandemic through the European Regional Development Fund (ERDF-OP 2014 – 2022) under the reference 2021 FE 9127.